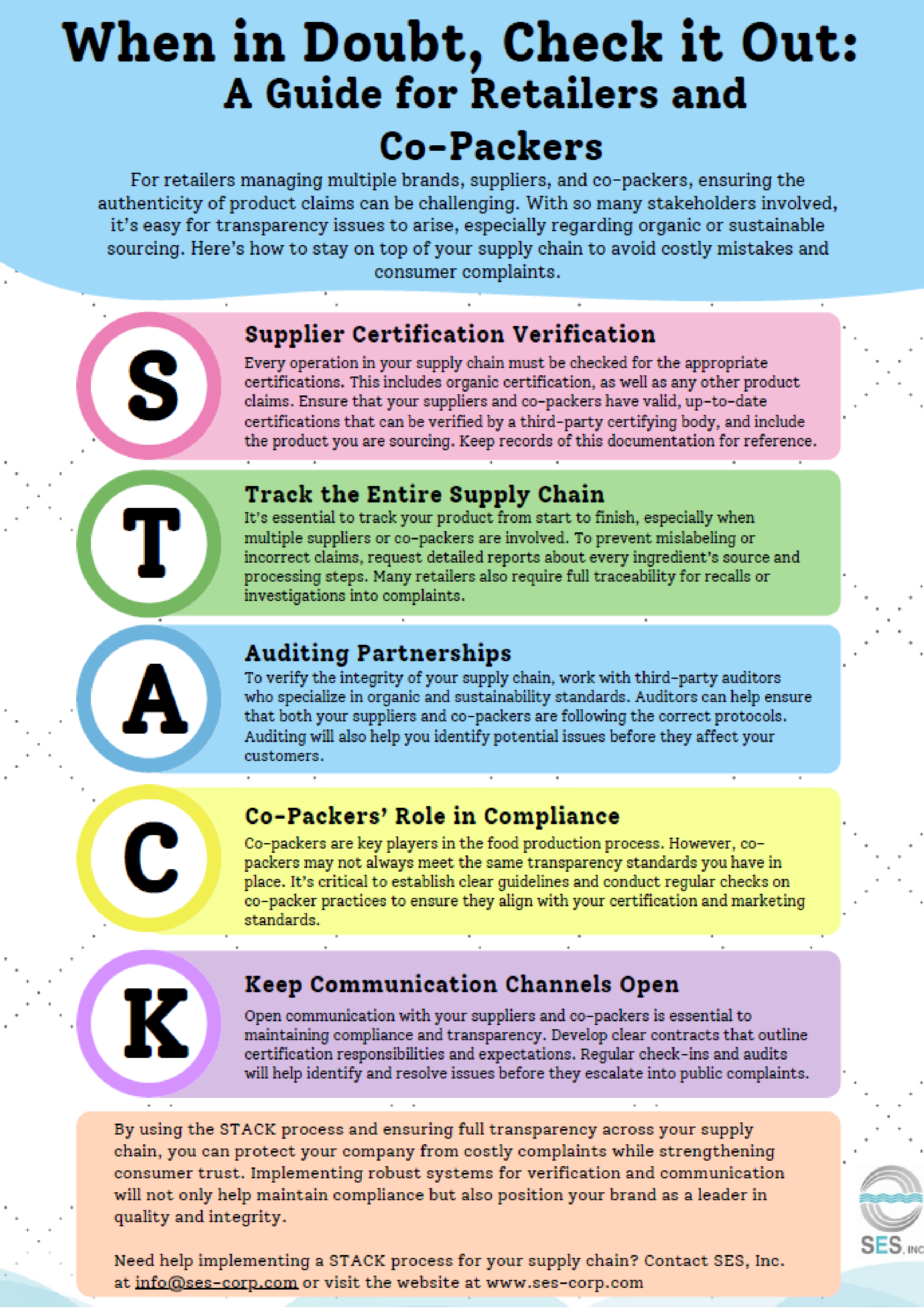
What the Food Traceability Rule Means for your Business
Today’s consumers are equipped with more food safety knowledge than ever. With technology, information on contaminated products is readily available to consumers instantaneously. Consumers are seeing the cause and effects of contaminated products that make it into the marketplace. Their purchasing behaviors are driven by a growing awareness of foodborne illnesses, contamination risks, and a desire for clean labels. Consumers want to see companies prioritize food safety and transparency in the food supply chain.
In response to growing food safety concerns, the U.S. Food and Drug Administration (FDA) enacted the Food Safety Modernization Act (FSMA) to transform our nation’s food safety system by shifting focus from responding to foodborne illness to preventing it. With this new act, the FDA has released numerous rules outlining clear and specific actions to prevent contamination. One of the rules released was the Requirements for Additional Traceability Records for Certain Foods (Food Traceability Final Rule), establishing extensive record keeping requirements for high-risk foods beyond the traditional scope of the regulations. The Food Traceability Final Rule requires those who manufacture, process, pack or hold foods to come into compliance with this rule by January 20, 2026. The following guidance can help your business meet the new requirements.
Need a quick way to create training and education for your team? Download this guide and helpful infographic.
Who does the rule affect?
Businesses who manufacture, process, pack or hold any of the following products on the Food Traceability List (FTL) are required to comply with Section 204:
- Cheeses (made from pasteurized milk) fresh soft, soft unripened, soft ripened, or semi-soft; cheese (made from unpasteurized milk) other than hard cheese
- Shell eggs
- Nut butters
- Fresh produce: cucumbers, leafy greens, leafy greens (cut), melons, peppers, sprouts, tomatoes, tropical tree fruits, fruits, and vegetables (cut)
- Finfish (fresh, frozen, and previously frozen); Smoked finfish (refrigerated, frozen, and previously frozen); crustaceans (fresh, frozen, and previously frozen); molluscan shellfish bivalves (fresh, frozen, and previously frozen)
- Ready-to-Eat deli salads
Implementing Section 204 will significantly impact the food industry. Companies must invest in updated recordkeeping and traceability systems to comply with the new regulations. This may involve adopting digital tracking solutions, enhancing supply chain transparency, and training employees on new compliance requirements. While these changes may present initial financial and operational challenges, they ultimately benefit companies by reducing the risk of large-scale recalls, protecting brand reputation, and enhancing consumer confidence. Improved traceability can also streamline supply chain management, reduce waste, and improve overall food safety standards.
Key Features of Section 204
- Critical Tracking Events (CTEs)
A fundamental aspect of Section 204 is the requirement to track CTEs throughout the food supply chain. CTEs include key points where food is grown, received, transformed, created, and distributed. These events ensure that all movements of food products are recorded, making it easier to trace the origin of contamination during a foodborne illness outbreak. By mandating comprehensive tracking of CTEs, the FDA aims to reduce the time required to remove contaminated food from the market and mitigate public health risks.
- Traceability Lot Code
The introduction of the traceability lot code is another essential requirement under Section 204. This unique identifier must be assigned to food items listed in the FTL and must follow the product throughout the supply chain. The lot code allows for quick identification of potentially contaminated food items and prevents widespread recalls that could affect unaffected batches. Companies are required to maintain records linking lot codes to CTEs, ensuring that in the event of an outbreak or contamination event, tracing back to the source is more efficient and accurate.
- Traceability Plan
Under Section 204, companies handling foods on the FTL must develop and maintain a traceability plan. This plan must detail the procedures and protocols for capturing traceability information, including how records are maintained, the types of data collected, and how businesses will respond to potential contamination events. The traceability plan must also identify the designated personnel responsible for compliance and provide training to ensure proper execution. This proactive approach ensures that businesses are prepared to respond to food safety incidents in a timely and effective manner.
- Additional Recordkeeping Requirements
In addition to the specific requirements outlined above, Section 204 mandates additional recordkeeping obligations for entities dealing with foods on the FTL. These include keeping electronic records that are easily accessible to the FDA upon request. The goal is to facilitate faster and more efficient investigations into foodborne illness outbreaks and contamination incidents. Companies must ensure that their recordkeeping systems are compliant with FDA standards and that data integrity is maintained throughout the supply chain. Failure to comply with these requirements can result in penalties and increased regulatory scrutiny.
Recommendations for Implementation
- Invest in Digital Traceability Solutions – Companies should transition from paper-based recordkeeping to digital systems that enable real-time tracking of CTEs and traceability lot codes. Blockchain and cloud-based solutions can enhance data security and accessibility.
- Develop and Regularly Update a Traceability Plan – Businesses should create comprehensive traceability plans that outline compliance procedures and response strategies for contamination events. Regular updates and audits will ensure continued adherence to FDA regulations.
- Train Employees and Suppliers – Ensuring that all stakeholders in the supply chain are well-versed in traceability requirements is crucial. Conducting training sessions for employees and working closely with suppliers to align practices can enhance compliance and efficiency.
- Perform Regular Compliance Audits – Routine audits will help identify gaps in traceability systems and allow for timely corrective actions. Companies should establish internal review processes and collaborate with third-party auditors to maintain high food safety standards.
Need Compliance Support?
SES offers a range of food safety services to help your business follow the FDA guidelines, ensuring integrity in your supply chain and maintaining transparency in marketing practices. Our services include supply chain outreach and audits, marketing consulting, and support with your HACCP, TACCP, and VACCP plans. Visit our website at https://ses-corp.com/food-integrity-and-safety/.
